

Elevate Your Learning Experience with Oter
Access to 17,000+ Books
Gain insights from a diverse range of subjects, enhancing knowledge and skills across various disciplines.
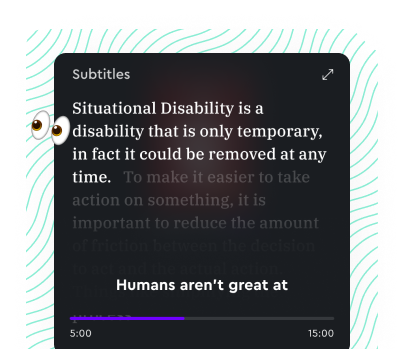
Learn on the go
Enjoy uninterrupted audio while multitasking. Follow along with real-time subtitles to enhance your listening experience.
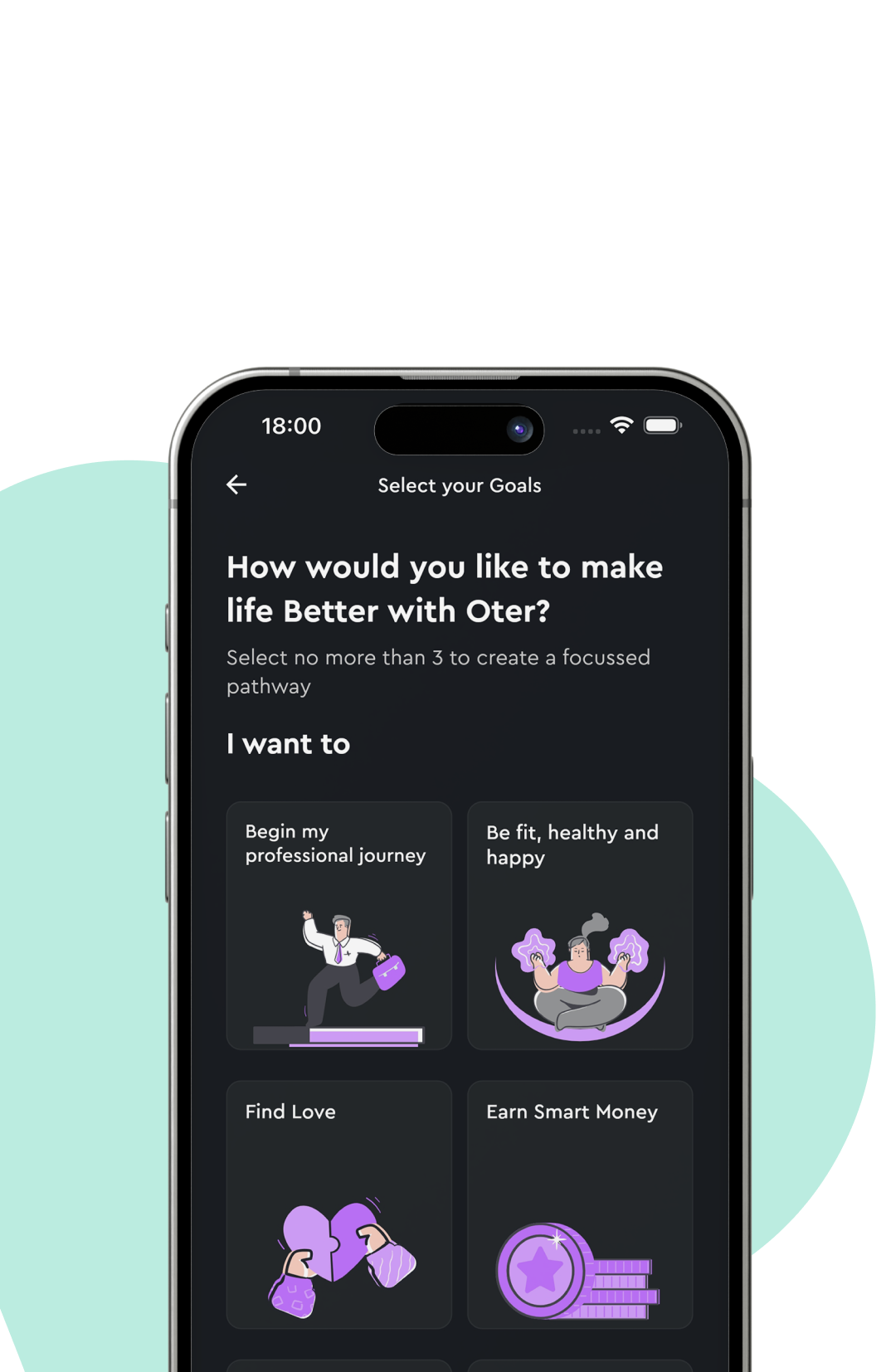
AI Recommendations
Discover book recommendations curated to align with your learning preferences and goals, ensuring every read supports your growth.
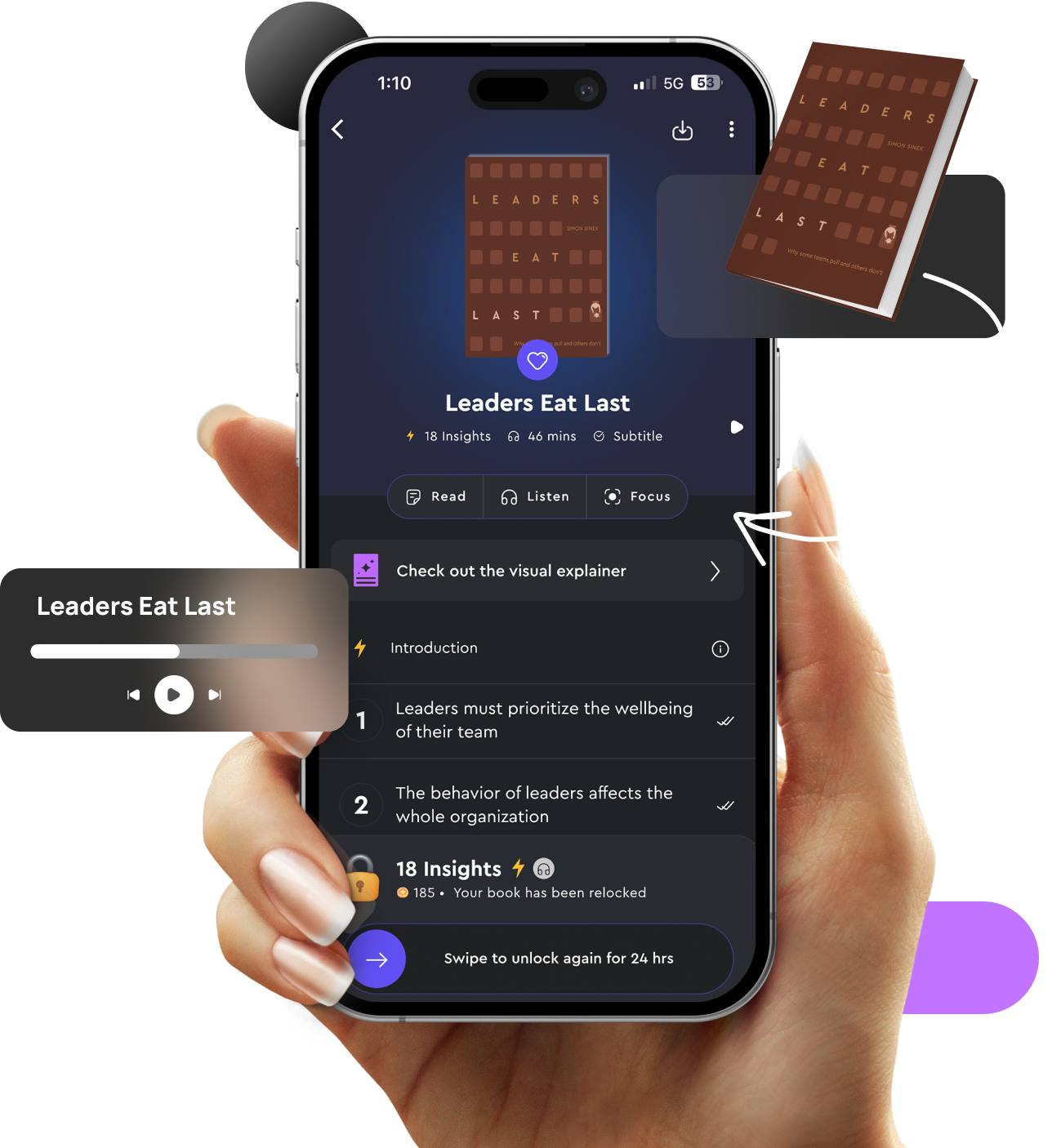
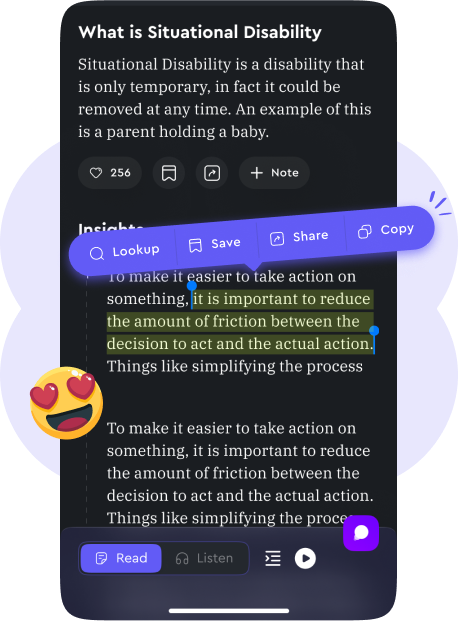
Take Notes, Highlight, and Save
Annotate and save important points for future reference, making it easier to review and apply insights.
Offline Access
Download Oter microbooks for offline access and learn on the go without needing an internet connection.
Badges and Streaks
Help yourself earn achievements by reading more, keep your streak going, and stay motivated.

17,000 Microbooks with Oter Pro and dive into endless learning
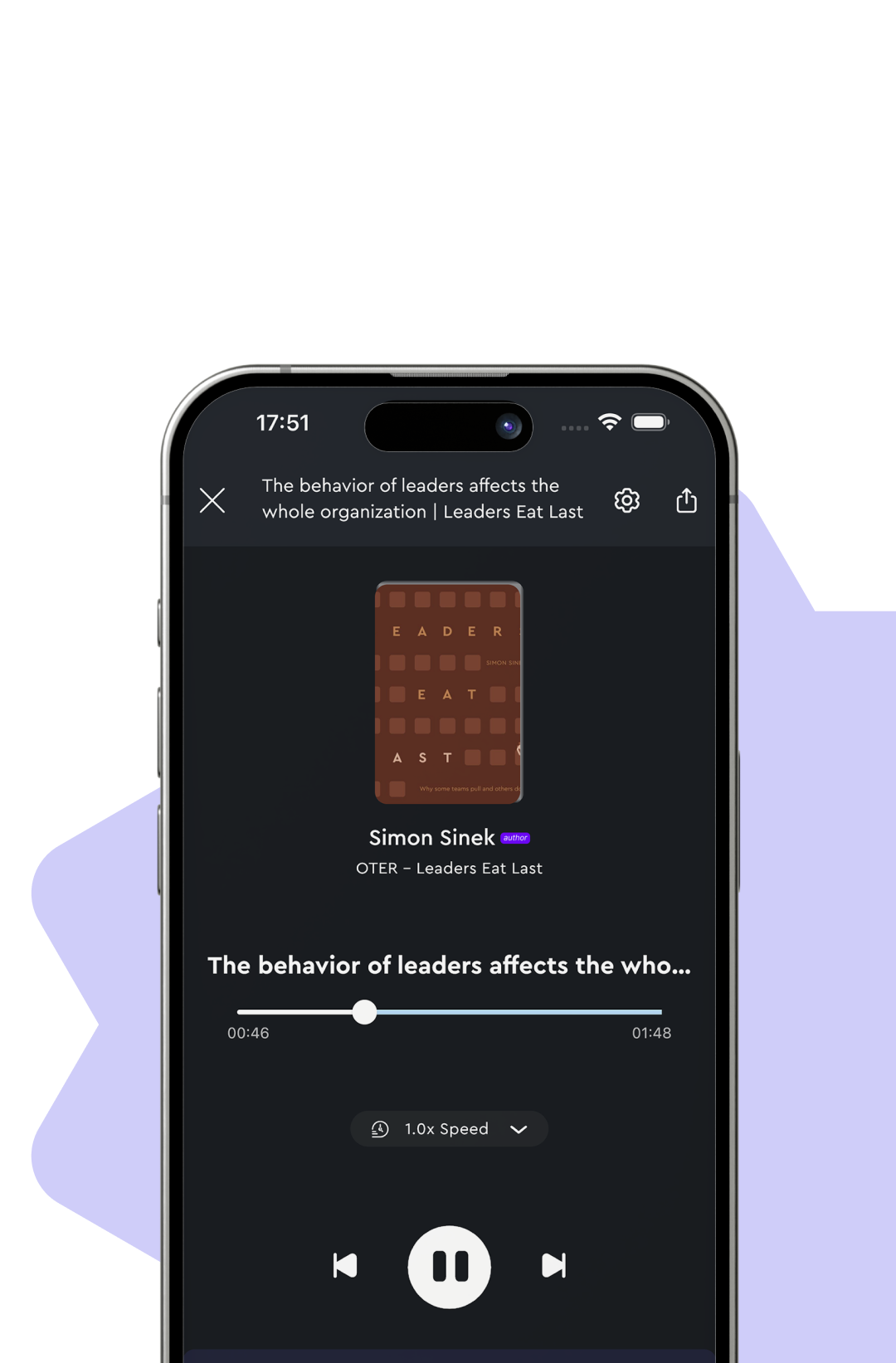

Audio on the Go
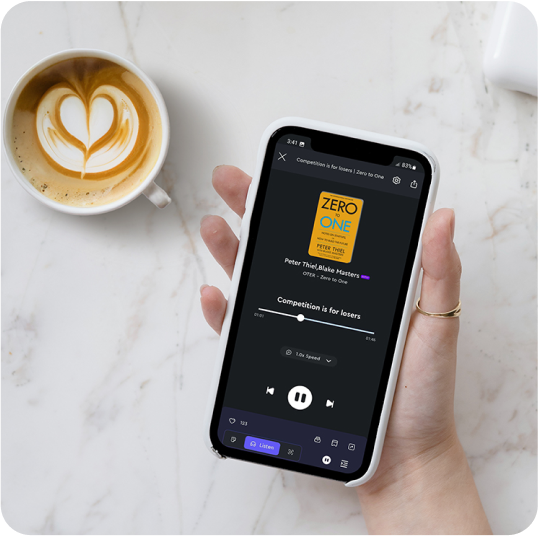
Have a coffee with OTER

Turn Work break into micro learning session

Listen & Learn while Driving
Join thousands reaching goals daily with Oter!
Adagboyi kelvin



It's a great app. Very accessible with lots of great books in all aspect of life.
Indonesia Sarva





The development of the app is totally great and the feature to select 10 books 📚 that's fantastic like people usually select many books then they don't even bother to come back and read them so it's like creating hype on them so they can open next day for more books 📚 …The app is great, fantastic, fabulous, amazing and there no words left..
C P Manna





Hey,your app is amazing! I loved it!! I have searched for books which I wanted to read and that should be free but couldn't find. But atlast through your app i'm able to read the books which I wanted. Thanks for creating the app which is helpful for everyone who wishes to read their desired book.
mehul shah





Excellent App with great attention to details. Life becomes easier and truly motivational.
Venkata amarnath





One and only great app for reading ♾️ Infinite number of books free of cost, and saving time by providing key points, This app helped me Alot , Also recommend to many people, LOVE 😘📖📖 This App.
Saikat Paul





This is an amazing app, and I have found it very useful in my daily life. With this app, I am sure that one can develop a strong reading habit in the long run. All the best!
Frequently Asked Questions (FAQ)
What is a Microbook?
Can I read Microbooks without a subscription?
How do credits on Oter work?
How can Oter help me in my self-improvement journey?